In 1898, Sir William Crookes[1] gave an inaugural speech as incoming president of the Royal Academy of Sciences. The opening line of his speech was – “England and all civilized nations stand in deadly peril of not having enough to eat.”[2] He continued with detailed data on wheat yields, imports, and rates of consumption, and he predicted that population growth would result in mass starvation by the 1930s. In his talk, Sir Crookes issued a challenge to his audience; he challenged those scientists listening to develop a method to mass-produce what he called “chemical manures” in order to avoid catastrophe.
This summer I became aware of work published by the Stockholm Resilience Centre at Stockholm University in Sweden.[3][4] Scientists at this Centre are attempting to identify and quantify the earth’s biophysical processes and their boundaries; asking how far they can be stretched before we can expect trouble to arise. Their research suggests that if we strive to keep our environmental impact within certain limits in specific key areas, we will reduce the risks of substantial and abrupt environmental shifts that would pose significant challenges to societies. Among the boundaries they have identified is the biogeochemical cycling of the elements nitrogen and phosphorus. Researchers conclude that our current practises exceed this boundary; our use of chemical fertilizers results in high levels of risk of harm to the environment and to human health.
Tonight, in my inaugural speech, I will tell the story of the birth of the chemical fertilizer industry, the enormous blessing it has been, its links to warfare, and the global and local environmental consequences of overuse of nitrogen fertilizers. I will conclude by suggesting practices that would allow us to reduce future risks to human health and to the environment.
This story is an excellent example of a complex modern issue that is a joy to teach about in a liberal arts and sciences setting. To understand the issue well, one needs to consider chemical, biological and environmental aspects of the story, but also historical, economic, social, political and theological aspects. The development and use of chemical fertilizers reflect our calling to unfold and develop creation and to care for the poor and hungry, while considerations of how to use this technology in ways that limit environmental harm stem from our calling to care for God’s good creation. As we shall see later, consistent with the research being done at the Stockholm, Resilience Centre, the Bible indicates that there are limits and boundaries within creation, and that it needs Sabbath rest and should not be pushed relentlessly.
Chemistry and Environmental Cycling of Nitrogen
Nitrogen was discovered in 1772 by Daniel Rutherford.[5] To unwrap this story I will first highlight some important aspects of the chemistry of nitrogen – which common forms of nitrogen exist, their properties, and how nitrogen can change from one form to another. I have long been interested in the boundaries between living things and their environment – how factors required to sustain life (or that can cause harm) behave in air, soil or water; how they are taken up and transformed by various organisms; and what role they play within living things.
While living things are made up of many elements, four predominate. Hydrogen and oxygen are present in large amounts in water and in many other biological compounds. Carbon is also common; it is the element that gives basic structure to almost all biomolecules. Carbon is abundant and easily accessible – plants get it from air as carbon dioxide, while animals get it from the carbohydrates, fats, and proteins eaten as part of their diets.
Living things require far less nitrogen than they do carbon, hydrogen, or oxygen. However, the presence of nitrogen helps to give biomolecules unique properties. Nitrogen is in DNA, which stores genetic information, and in the amino acids needed to make proteins, which make up muscle tissue and the countless enzymes that regulate the rates of the many chemical reactions within cells that sustain life. In chemical terms, carbon has been described as providing the quantity of life, while nitrogen has been described as giving the quality of life.
Within the environment, nitrogen takes a number of different forms (Table 1). Approximately 78% of the air that we breathe is made up of nitrogen gas (N2), a rather non-reactive molecule made up of two nitrogen atoms bound very tightly together. Other gaseous forms of nitrogen include nitrous oxide (N2O), which is a greenhouse gas, ammonia (NH3), and nitrogen oxides (NOx), air contaminants that can cause acid rain and ground-level ozone. Nitrogen dissolved in water (including soil water) is present as the ions ammonium (NH4+) and nitrate (NO3–). Finally, nitrogen is present in many important and complex biomolecules including amino acids, proteins and nucleic acids.
Table 1: Some nitrogen-containing molecules within creation.
| molecule | chemical formula | role/function/characteristic |
| nitrogen gas | N2 | – a gas; ~ 80% of atmosphere; quite inert (non-reactive) |
| nitrous oxide | N2O | – a greenhouse gas; ~ 200x more potent than carbon dioxide |
| nitrogen oxides | NOx (NO & NO2) | – gases; can be air pollutants; result in ground-level ozone and acid rain |
| ammonia | NH3 | – a gas; a ‘fixed’ form of nitrogen |
| ammonium | NH4+ | – an ion found in soil or water; a ‘fixed’ form of nitrogen – bioavailable |
| nitrate | NO3– | – an ion found in soil or water; a ‘fixed’ form of nitrogen – bioavailable |
| glycine | C2O2H5N | – one of 20 amino acids; building blocks for proteins (which are ~ 16% nitrogen) |
| biomolecules | varies | – nucleic acids store and transmit information, proteins catalyze reactions, others… |
The biochemistry of nitrogen – that is, how living things access and utilize it – is a complicated but important part of this story. In creation, most nitrogen exists as nitrogen gas in the air, but most living things cannot use this form of nitrogen. People (eating a diet with inadequate levels of protein), or plants in a garden (lacking sufficient levels of bioavailable soil nitrogen) can be deficient in nitrogen, even though it is in the air all around. Plants predominantly take up nitrogen if it is in soil in the form of nitrate or ammonium. Plants and bacteria have ways of converting nitrate to ammonium and vice versa, so both these forms of nitrogen are considered bioavailable. Once inside plant cells, nitrogen (in the form of ammonium) can be incorporated in to proteins, and the nitrogen can then move up food chains when plants are eaten by animals that are then eaten by other animals.
Globally, nitrogen is the plant nutrient that is most commonly limiting; in many ecosystems around the world, both natural and agricultural, a lack of bioavailable nitrogen limits production or yield. This becomes evident if you try gardening or farming without any outside source of nitrogen. Throughout all of history, whether farmers knew it or not, farming has in part been about increasing the amount and bioavailability of nitrogen in soils. Manures from humans or other animals (e.g. cows and chickens), and composts made from decayed plants are good sources of nitrogen that have been used for thousands of years to maintain soil fertility.
An essential piece of nitrogen biochemistry is called biological nitrogen fixation. This process is naturally done by a select few species of bacteria and fungi, most of them associated with legumes plants such as clover, beans and peas. Bacteria living in a symbiotic relationship with these plants have the unique ability to convert inert nitrogen gas to reactive ammonium, a bioavailable form of nitrogen (Fig. 1). They do so by using copious amounts of energy (supplied by the plants) to break apart molecules of nitrogen gas. The plants get the fixed nitrogen, and the bacteria get energy in the form of sugars that plants make from sunlight. Natural ecosystems contain nitrogen-fixing plants. All over the world, legumes have been, and still are used in crop systems to maintain soil nitrogen. The Romans grew wheat after a crop of beans, and noted improved yields. Farmers here in Ontario grow corn after soybeans. Clover is included in pastures and hayfields. Farmers in parts of the tropics use velvet bean as a cover crop to conserve soil moisture, to build up soil organic matter and to replenish soil nitrogen.
Figure 1: Nitrogen-fixing nodules on soybean.[6]
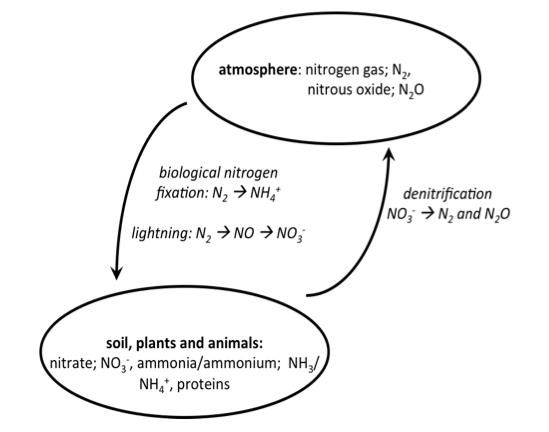
To recap nitrogen chemistry and its movement – in air it is present as nitrogen gas (Figure 2). It can be fixed (by legumes) to ammonium ions (NH4+) or by lightning to nitrate ions (NO3–). In soil it can be converted between ammonium and nitrate and incorporated into proteins by soil microbes and plants. Animals consume nitrogen in the form of plant or animal proteins as part of their diet. Plant composts and animal wastes cycle nitrogen back into soil. And, finally, by a process called denitrification, soil nitrogen can be lost to the atmosphere through the activity of certain soil microbes that can convert soil nitrogen back into nitrogen-containing gasses.
Figure 2: Nitrogen cycling between the atmosphere and land.
Some History
As mentioned, an important part of farming is maintaining and building up soil nitrogen, in order to maintain the health of plants and of the people and other living creatures consuming the plants. This was, and still is, done by using legumes in crop rotations; by incorporating animal manure, composts, and plant biomass (especially of legumes) back into soil; or by farming newly cleared forest or grassland soil (i.e. slash and burn). All over the world, farming systems evolved that used locally adapted methods, plants and animals to maximize production. In parts of China and other regions in Asia, where farming has occurred continuously for thousands of years, farmers used a carefully designed system that was probably the highest-yielding system developed prior to the 1900’s. It included the practise of carefully collecting all human waste for addition to soils of neighboring farms.[7]
Here in the west, we have a globalized food supply system. Almost any type of food is available at any time, as long as you have money to buy it. Traditionally, however, whatever could be grown in a particular region had to keep a local population fed. This meant that a population was dependent on local crop yields, and crop failures resulted in famines. Famines were not uncommon. In Joseph’s time, Egypt had a tough seven years of famine, and Ruth the Moabitess married an Israelite whose family fled to her country to avoid famine. In the 11th century, France experienced 26 famines[8], though the frequency of famines in Europe gradually decreased as newer farming techniques were developed, as trade increased, and as nation states gradually became established.
In Europe by the early- to mid-1800s, most available farmable land had been cleared.[9] Despite many wars, plagues, and famines, population had slowly grown until it was evident that population growth was straining the ability of European soils to provide enough food. Prior to modern times, there was a limit to the number of people good arable land could support. Vaclav Smil has presented data suggesting that environments as diverse as the Netherlands, Egypt, Java, and China could sustainably support population densities of only four to five people per hectare of arable land.[10] As population densities in Europe increased, additional food had to be supplied by imports from Russia and from colonies overseas.[11]
By the mid-1800’s, in an effort to increase crop yields, European nations discovered and began to exploit sources of nitrogen far from Europe. In a curious twist of geography and ecology, some islands (called guano islands) off the coast of Peru were discovered to have enormous deposits (up to 50 m deep!) of guano (i.e. bird droppings) rich in nitrogen and phosphorus (Figs 3 A and B).[12] The islands are important bird nesting sites and the guano built up over time. Because there is so little rain, the guano is not washed away and it simply collects over time. At the peak of the guano trade, over 500 000 tonnes per year was exported from Peru.[13] The importance of this resource is reflected by the creation of the Guano Islands Act, passed by US Congress in 1856 which allowed US citizens to claim possession of uninhabited guano islands.
Figures 3 A and B: Guano Islands off the coast of Peru.[14]
European dependence on far-away guano reminds me of the West’s current dependence on oil from the Middle East; there is nothing new under the sun. The guano was extracted at an unsustainable rate. As a resource, it did not last for long, and by the 1860s-70’s, production began to decline. The race for the next source of nitrogen was on.
It was found not far away in the Atacama Desert on the west coast of South America. Because nitrate is so soluble in water, minerals of nitrate are rare; with rainfall, nitrate salts will simply dissolve and leach away. However, the Atacama Desert is so dry that the mineral sodium nitrate (Chilean saltpeter) is common, and can easily be surface-mined. A second nitrogen boom followed. Nitrate mining towns popped up, followed by a border dispute and then war between Chili (on one side), and Bolivia and Peru (on the other) in the late 1870s. As with the guano, exports of Chilean nitrates rose to almost 500 000 tonnes per year in the years leading up to WWI.[15]
This finally brings us back to Sir William Crookes, who I mentioned at the opening of this talk, and his challenge to develop a method to mass-produce “chemical manures” to avoid catastrophe. Scientists in Britain, France and Germany took up the challenge and began to work out methods to synthesize ammonia – to essentially replicate, in a chemistry lab, what legumes do naturally. The effort was greatest in Germany, perhaps in part because of a realization that the nation was vulnerable to blockades which could reduce imports of food and/or nitrates in times of war.
Fritz Haber (Fig. 4A) was a German Jewish scientist. In 1909 after much effort, Haber determined that in the lab, a mixture of nitrogen from the air, hydrogen gas (made from the electrolysis of water), and a catalyst heated to 600oC at 200 atmospheres of pressure would produce ammonia. He partnered with a young chemical engineer named Carl Bosch (Fig. 4B) who worked at a German chemical firm called BASF[16] that had been formed in the 1800s to produce synthetic dyes. After much trial and error, Carl Bosch oversaw the development of the necessary infrastructure and methods to produce ammonia on an industrial scale. The process, still in use today, is called the Haber-Bosch process, and it has been called by some the single most important piece of technology developed in the 20th century – even more important than flight or computers.[17] The method has been described as a way to allow us to turn air into bread. Every introductory chemistry textbook that I’ve ever come across uses the reaction to illustrate concepts of equilibrium chemistry, though almost none address the history or significance of the development.
Figure 4 A and B: Fritz Haber and Carl Bosch[18]; Haber process (N2 + 3 H2 à 2 NH3)
For his work, Fritz Haber was awarded a Nobel Prize in Chemistry in 1918. Carl Bosch was co-awarded a Nobel Prize in Chemistry in 1931 for his work in developing high-temperature and high-pressure methods in chemistry.
The award to Fritz Haber was not without controversy. During the early decades of the 1900’s, some Jews (including Haber) did their best to fit into German society and prove their dedication to the nation. During WWI, Haber threw himself into poison gas research, and personally directed poison gas attacks. In horror, his wife committed suicide. After the war, Haber tried unsuccessfully to develop a method to filter gold out of seawater to pay German reparations. In a horribly ironic twist of history, his research into poison gas was built upon to develop the gasses used to poison Jews in the gas chambers during WWII. Haber died in exile in Switzerland in 1933.
Impact on War
Thus far, I have referred to the use of guano and Chilean nitrates for fertilizers. However, nitrates have another important use. As Europeans grew to rely more and more on guns and cannons throughout the 15th to the 19th centuries, more and more gunpowder had to be manufactured. Gunpowder (black powder) was made from three ingredients: 15% charcoal (relatively easily made), 10% sulfur (mined and processed by a messy process in volcanically active areas), and 75% saltpeter (potassium nitrate). When ignited, a large volume of hot gas is quickly produced by the reaction below, which is able to propel the projectile.
8 C(s) + 3 S(s) + 10 KNO3(s) à 2 K2CO3(s) + 3 K2SO4(s) + 6 CO2(g) + 5 N2(g)
carbon (charcoal) + sulfur + potassium nitrate (saltpeter) -> potassium carbonate + potassium sulfate + carbon dioxide gas + nitrogen gas
Mineral sources of nitrates were rare, so nitrate was made via a tedious process that involved collecting animal manure and/or human urine, and composting the mixture to allow the nitrogen to be converted to nitrate. The nitrates were then leached out and precipitated, purified and concentrated (Figure 5). Saltpeter was in such demand that in 1626, Charles I of England demanded all citizens collect urine (their own, and that of their animals) for this process, and saltpeter men were hired to enforce this rule.[19]
Figure 5: An engraving from the 1500’s illustrating saltpeter production.[20]
In 1867, Alfred Nobel invented dynamite (Figure 6 A and B), an explosive many times more powerful than gunpowder. The synthesis of nitroglycerin, the active ingredient, requires reactive nitrogen. The manufacturing and use of dynamite resulted in advances in mining techniques, the creation of canals (Suez and Panama) and railway tunnels, all of which contributed to the development of modern society. It also led to the production of more powerful explosives for use in war. Alfred Nobel became very wealthy, and somewhat conflicted about the use of his invention. Upon his death he left instructions to use his fortune to fund prizes; to this day the trust fund awards five Nobel prizes of ~ 1 million dollars each year.[21]
Figure 6: Alfred Nobel[22], nitroglycerin, and dynamite.[23]
Increased demand for dynamite resulted in increased demand for nitrates. Production of saltpeter in India fueled trade and contributed to the eventual colonization of India. The discovery of the guano islands, and then Chilean nitrates, drastically increased access to the raw materials needed to make explosives, but also made nations dependent on regular imports that could be disrupted in times of war – precisely when supplies were most needed.
Very likely, Haber’s motives for finding a way to synthesize ammonia were mixed. While it would be helpful to secure a source of nitrogen fertilizer that could not be blockaded, securing a source of fixed nitrogen to make explosives would serve the nation even more during an unsettled time politically.
When World War I broke out, many Germans expected (or at least hoped) that war would be brief; in any case Germany had stockpiled about six months worth of explosives, or the raw materials needed to synthesize them. It quickly became clear that a large supply of nitrates would be critical to maintain the war effort. The first major naval battle of the war was fought off the coast of Chile, where both Germany and Britain were trying to protect their access to Chilean nitrates. Germany won the first battle, but the British quickly gained control of the Chilean saltpeter trade, and Germany was largely shut off from nitrate imports. At the same time, on the home front, the new BASF ammonia production plant was converted to produce nitrate – the same compound that was being imported from Chile, and that could be used in the synthesis of explosives. BASF, nitrogen, and the discipline of chemistry were now part of the defense industry. The technological advances extended the war by years; without the ability to fix nitrogen synthetically, Germany would have very quickly run out of explosives.
Impact on Agriculture
The impact of the Haber-Bosch process on agriculture cannot be understated. Haber’s and Bosch’s achievements meant that Sir William Crookes’ prediction of widespread famine did not occur. To those who had access to the new nitrogen fertilizers, it was relatively easy and inexpensive to maintain sufficient levels of nitrogen in soil. Plants could now be bred that could utilize essentially unlimited pools of nitrogen. If the plants could allocate more of their growth into seed, and less into roots or stems, yields could be steadily increased. The combination of chemical fertilizers, irrigation techniques, pesticides, and newly bred plant varieties (bred to use these new resources) together resulted in the Green Revolution. Beginning with corn in the 1930’s, the revolution spread to wheat and rice soon after; these three crops supply most of the necessary calories to the world’s population. The results have been astounding. From the mid 1800’s until the mid 1930’s, US corn yields hovered around 20-30 bushels per acre. Since the 1930’s, yields have steadily climbed all the way to 150-160 bushels per acre, a six-fold increase in yield; this is equivalent to about 10 tonnes per hectare. However, we may be reaching the limit in terms of what maximal yields are possible with some of the major world crops. Wheat yields in parts of Europe have plateaued at seven to eight tonnes per hectare. Rice yields in Japan, at just under five tonnes per hectare, have not increased in almost 20 years. In recent years, rice yields in China have approached those in Japan, and may also be about to plateau there as well.[24]
In short, nitrogen fertilizers are partially responsible for the fourfold increase in population of the last hundred years (Fig 7). We are now quite dependent on nitrogen fertilizer to feed the world’s 7.3 billion people. Vaclav Smil has estimated that approximately 40% of the world’s population is fed by nitrogen fertilizers; put another way, approximately 40% of the nitrogen in our bodies has flowed through a chemical fertilizer plant operating the Haber-Bosch process on a large scale.[25] Where population densities used to hover around four or five people per hectare of good arable land, the use of nitrogen fertilizers and modern cultivars of major crops enables 15 to 20 people to be fed per hectare of arable land.[26]
Fig 7: Global population growth.[27]
Environmental Impact
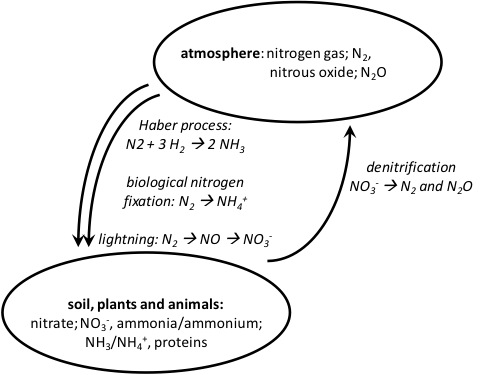
Today, the Haber-Bosch process is operating on a massive scale. In total, over 400 billion kg of nitrogen are fixed (and moved from the atmosphere to land and water) each year.[28] More than half of this is moved as a result of human activities. About 120 billion kg (almost 30%) flows through Haber-Bosch fertilizer plants;[29] this amount equals all natural biological fixation on land by all natural and agricultural systems on the planet. We have substantially added to the natural nitrogen cycle (Fig. 8).
Figure 8: Modern nitrogen cycling between the atmosphere and soil.
Most newly adopted technologies have unintended consequences. I am reminded of a tongue-in-cheek quote by Wendell Berry: “The smartest and most educated people are the scientists, for they have already found solutions to all our problems and will soon find solutions to all the problems resulting from their solutions to all the problems we used to have”.[30] This is where we are now at with our use of nitrogen fertilizers; in averting Sir William Crooke’s predicted famine of the 1930’s, we’ve created a nitrogen pollution problem that needs to be addressed.
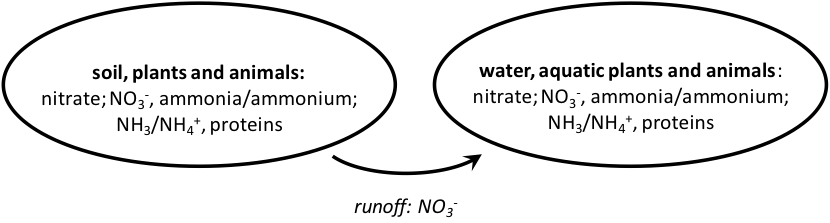
So what are the issues caused by our global use of nitrogen? In an ideal world, soils deficient in nitrogen can be supplied with just enough nitrogen to be taken up by plants. In reality, nitrogen is applied in excess, to ensure maximal yield. Galloway and Cowling have estimated that only a small fraction (4-14%) of the nitrogen applied as fertilizer actually makes it into the food we eat.[31] There are three fates for excess nitrogen that is not taken up by plants. First, it can undergo a process called denitrification by which soil microbes convert nitrate into gaseous forms of nitrogen. Second, it can leach into ground water and contaminate wells with excess nitrates. Third, it can enter creeks and streams contaminating surface waters or the ocean (Fig. 9).
Figure 9: Nitrate run-off from land into aquatic systems.
One of the gases formed by denitrification is nitrous oxide (N2O), a greenhouse gas that is about 200 times more potent (molecule by molecule) than carbon dioxide. According the USEPA, nitrous oxide emissions have increased by 8% between 1990 and 2013, and three quarters of all nitrous oxide emissions are due to agricultural activities.[32] Our use of nitrogen fertilizers is directly linked to increasing concentrations of greenhouse gasses and human-caused climate change.
Excess nitrates in groundwater become a concern when these sources are tapped for drinking water. Once ingested, some of the nitrate is converted to nitrite. Nitrite reacts with hemoglobin and interferes with its ability to bind to oxygen and deliver it to tissues. One symptom of over-exposure to nitrate is shortness of breath. In very young children, overexposure results in a disease called “blue baby syndrome”.[33]
Many countries have set 10 ppm nitrate-N as the maximum acceptable level of nitrate in drinking water. In the US, in areas with high nitrogen fertilizer use and well-drained soils, almost one quarter of shallow wells exceed drinking water quality guidelines.[34]
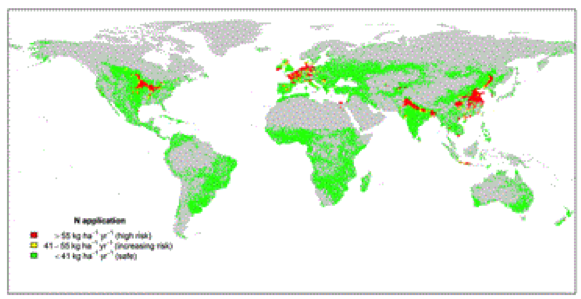
Probably the largest environmental impact of overuse of nitrogen is on surface waters. Aquatic life is adapted to water with low levels of nitrogen and of other nutrients such as phosphate. When nitrogen and phosphate, from agricultural practices or from sewage run-off, are added to surface waters, a process called eutrophication occurs. Natural populations of algae grow rapidly (or bloom), resulting in murky green water. The algae eventually die and begin to decay, a process that removes oxygen from the water. Water becomes low in dissolved oxygen or even lacking oxygen, and unable to support fish life. Lake Winnipeg in Manitoba, the Great Lakes in Ontario (especially Lake Erie), and Cootes Paradise here in Hamilton are stressed. Vast swaths of the Gulf of Mexico at the mouth of the Mississippi are so anoxic they are unable to support life (Figure 10 A and B).
Fig 10 A and B: Nitrogen application rates[35], and sites where eutrophication is occurring.[36]
Waterfalls Project
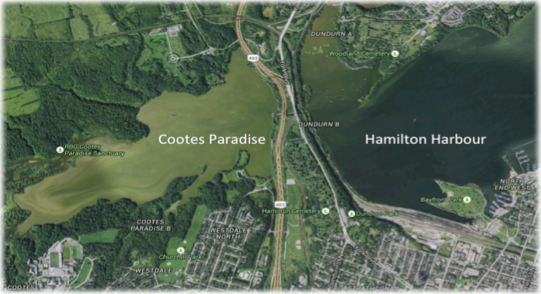
This past summer, Dr. Darren Brouwer and I received funding from Redeemer’s Center for Christian Scholarship that allowed us to hire students to monitor water quality of local watersheds flowing into Cootes Paradise (Fig 11 A and B).
Fig 11 A and B: Map of Cootes Paradise and Hamilton Harbour[37], and water research sampling sites.
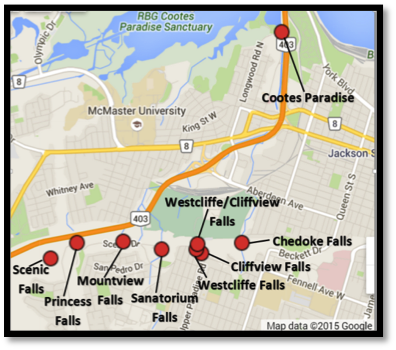
Our main focus was on the Chedoke Creek subwatershed, though we also sampled water from the Tiffany Creek, Ancaster Creek, and Spencer Creek subwatersheds, and other more minor creeks flowing into Cootes Paradise. Some of the nitrogen and phosphate is probably coming from agricultural sources. However, the most contaminated samples were from urban creeks thought to have sewage entering them. Specifically, some of the beautiful waterfalls of the Chedoke Creek subwatershed that are flowing over the escarpment nearby have levels of nitrogen and phosphorus high enough to cause eutrophication (Figure 12).
Figure 12: Nitrate-N concentrations in the Chedoke watershed.
It will take a considerable amount of money and effort to precisely identify the exact sources of the nitrate and to correctly plumb older neighbourhoods to limit the amount of nitrogen and phosphorus flowing into local water. In the long run, we will need to revisit our use of clean water to pipe away nitrogen-rich sewage waste. We need a modern-day Charles I to lead us in a redesign of our sewage infrastructure that will enable us to recapture nitrogen – not for making gunpowder, but to protect aquatic ecosystems and to recycle our nitrogen fertilizer.
Summary/conclusion
God created and upholds the chemical and biological laws for creation, and nitrogen is subject to them. Scientists, engineers, and farmers have developed the power to fix nitrogen and use it to significantly increase crop yields. However, we have been impatient and greedy, and we are starting to realize the results of our actions.
It is not easy to tease out an appropriate response to our current situation. We are called to serve the needs of others, especially the poor and vulnerable who do not have enough to eat. We are also called to steward the creation – to, in the words of Andy Crouch, help the cosmos “… groan a bit less and sing a bit more”.[38]
During the last hundred years, humans have modified the natural nitrogen cycle on an enormous scale. This has resulted in food security for more people than at any time in history. Food is so affordable that Canadians and Americans spend less than10% of their income on food.[39] Significant progress has been made toward achieving one target of the UN’s Millenium Development Goals: the proportion of undernourished people in the developing world has fallen from 23.3 to 12.9% in the last 25 years.[40]
Much of the progress has occurred in China, which has rapidly increased its use of chemical fertilizers since the 1970’s. A great many people in south Asia, sub-Saharan Africa and elsewhere desperately need more food security. This is a critical concern, given that population is expected to increase from our current 7.3 billion to 9-10 billion in the coming decades, and that much growth will occur in areas currently lacking adequate food security. Chemical fertilizers have played a role in these efforts, and will continue to do so.
However, these advances in food security have come at a serious environmental cost. When soil is treated as a machine to which we add nitrogen and from which we withdraw crops, rather than the complex inter-connected medium it truly is, it is damaged over the long term. Inherent soil fertility declines, rates of erosion increase, the risk of climate change increases, and surrounding water is contaminated – to the peril of our own health and the health of the environment as a whole.
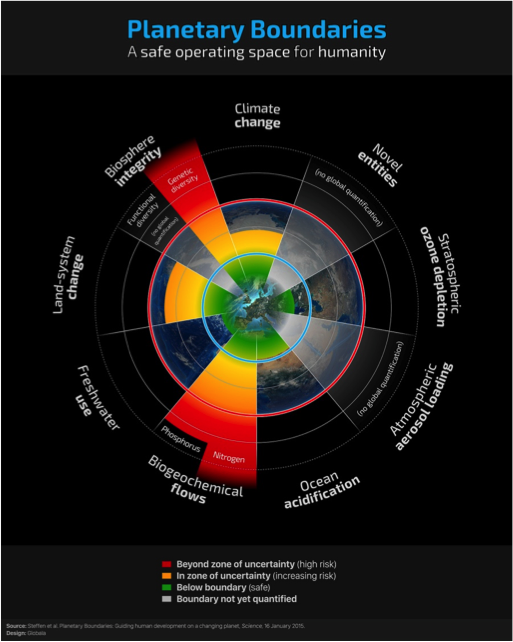
At the beginning of my talk I mentioned the Stockholm Resilience Centre (Stockholm University), where scientists studies social-ecological systems with a focus on the concept of resilience – the ability of a system or society to deal with change and to continue to develop.
Researchers at the Centre have identified nine planetary boundaries within which humanity needs to operate; examples include biodiversity loss, stratospheric ozone depletion, climate change, and, biogeochemical flows, which refer to the global movement of nitrogen and phosphorus (Fig 13). Researchers have concluded that we are operating within safe limits for some boundaries (e.g. ozone depletion), and that for others, insufficient data prevent us from knowing how to quantify the current reality and how to know what are appropriate limits (e.g. atmospheric aerosols). However, for other boundaries, including those associated with nitrogen and phosphorus cycling, evidence suggests that we have exceeded ‘safe’ boundaries. As a result, we face increased risk of abrupt or irreversible changes in the oceans, and in local watersheds, that will diminish the degree to which creation as a whole, including humankind, will be able to flourish.
Fig 13: Planetary boundaries.[41]
When I read this research, I am reminded of the Biblical concept of giving the creation Sabbath rest, and to not push it so relentlessly. Leviticus is full of such beautiful versus. For example, Lev 25:1-5[42] reads:
“The Lord said to Moses on Mount Sinai, “Speak to the Israelites and say to them: ‘When you enter the land I am going to give you, the land itself must observe a Sabbath to the Lord. For six years sow your fields, and for six years prune your vineyards and gather their crops. But in the seventh year the land is to have a Sabbath of rest, a Sabbath to the Lord. Do not sow your fields or prune your vineyards. Do not reap what grows of itself or harvest the grapes of your untended vines. The land is to have a year of rest.”
Ezekiel 34:18 shares a picture of careless and harmful treatment of the land, teaching that while we are called to live in the land and take what is needed, we are not to do so in a way that damages it for others:
“Is it not enough for you to feed on the good pasture? Must you also trample the rest of your pasture with your feet? Is it not enough for you to drink clear water? Must you also muddy the rest with your feet? Must my flock feed on what you have trampled and drink what you have muddied with your feet?”
Given what we know now about nitrogen we could say “Is it not enough to make fertilizers and grow good crops? Must you also fowl local wetlands with too much excess nitrogen? Must my other creatures suffocate from lack of oxygen?”
There are norms for the use of nitrogen we should follow, and if we don’t follow them, we can expect problems to arrive. Creation is good, and beautiful, and interconnected, and complicated. We all must strive to do what we can to obey God’s commands to care for the earth He made.
While I do not have expertise in the area of developing policy, I will offer a few thoughts; some top-down, and others bottom-up.
Policies will need to be developed that utilize the market. For many of our environmental issues, the environmental costs of using a particular resource are not counted in the price. The costs of poor drinking water quality, climate change, and degraded aquatic ecosystems are not born by those buying fertilizers or food, but somehow theses costs should be included. Economic signals could result in practices that change how nitrogen fertilizers are used. If quotas were issued to limit the total amount of nitrogen applied in certain regions, then farmers would be able to determine how and where to apply to get the biggest return.
I think that complex global issues are created, and perhaps also addressed by countless local decisions. How will cities address sewage treatment? Are there food policies that promote agricultural practices that result in less nitrogen waste? What types of food will consumers purchase? What about food waste? It seems to me it is a sin that roughly 40% of food, grown with much energy and nitrogen fertilizers, is simply wasted.
At its root, we are called to care about these issues. I am reminded of this quote from the end of the Lorax, by Dr. Seuss: “Unless someone like you cares a whole awful lot, nothing is going to get better. Its not.”[43]
There are encouraging signs of change. There is a growing permaculture movement in which food is produced in permanently planted integrated systems which do not depend on as much nitrogen inputs. Farmers in Kenya have used small amounts of nitrogen fertilizer applied in trace amounts (by the bottle-cap!) precisely where it can have the greatest impact on yield. There is a growing interest in backyard gardening using compost as the main source on nitrogen.
You now know hopefully more about nitrogen fertilizer than when you came. With knowledge, comes a responsibility to act. While it is easy to become discouraged by the scale of this issue, it is important to remember that God calls us to be faithful, not to succeed in solving the world’s nitrogen problems.
So, how can I respond? Can I reduce my nitrogen fertilizer footprint? If I consider the 140 billion kg of nitrogen turned into fertilizers, and assume that I am part of the wealthy 15% of the world that consumes 80% of its resources, my contribution is about 100 kg nitrogen/year. If I can cut this in half, by eating less meat, or by gardening without chemical fertilizers or by buying organic food, I can cut the need for global nitrogen fertilizer use by… 0% (0.00004%).
Perhaps I can inspire classes full of students to follow my lead, or influence Redeemer as an institution to purchase local, organic food, or even grow some of its own food on some of the great soil we have on campus.
As Christians, we are all too aware that we live in a fallen world. But, we also know that God created everything good, and that he still claims it as His own. God established His covenant long ago with Noah, and all of the other creatures. He told Noah:
“I now establish my covenant with you and with your descendants after you and with every living creature that was with you—the birds, the livestock and all the wild animals, all those that came out of the ark with you—every living creature on earth.” (Genesis 9:9-10)
Centuries later Christ summarized the commands as “love the Lord your God …” and …”love your neighbour as yourself.” One way we can try to obey these commands is to live in harmony with creation, including the nitrogen cycle, and try to undo some of the damage that has been done. Christ died and will come again to restore the whole creation. In the meantime, we seek to live justly, with kindness, as we walk with our God.
In one of my favourite paintings, Rembrandt imagined Christ embarking on this work as a gardener (Fig 14). We can do these tasks with hope and with joy.
Thank you for coming, and for this opportunity to speak this evening.
Figure 14: The risen Christ appearing to Mary Magdalene.[44]
References
Berry, W. 1992. Sex, Economy, Freedom & Community. Pantheon Books, New York, NY, USA.
Bown, S.R. 2005. A Most Damnable Invention: Dynamite, Nitrates, and the Making of the Modern World. Thomas Dunne Books, New York, NY, USA.
Brown, L.R. 2012. Full Planet, Empty Plates: The New Geopolitics of Food Scarcity. W.W. Norton & Company,, New York, NY, USA.
Crouch, Andy. 2013. Playing God: Redeeming the Gift of Power. IVP Books, Madison, WI, USA.
Fowler D., Coyle M., Skiba U., Sutton M.A., Cape J.N., Reis S., Sheppard L.J., Jenkins A., Grizzetti B., Galloway J.N., Vitousek P., Leach A., Bouwman A.F., Butterbach-Bahl K., Dentener F., Stevenseon D., Amann M. and Voss M. 2013. The global nitrogen cycle in the twenty-first century. Philosophical Transactions of the Royal Society B 368: 20130164.
Galloway JN. And Cowling EB. 2002. Reactive Nitrogen and the World: 200 Years of Change. Ambio, Vol. 31, No. 2, Optimizing Nitrogen Management in Food and Energy Productions, and Environmental Change, pp. 64-71.
Hager, Thomas. 2008. The Alchemy of Air. Three Rivers Press, New York, NY, USA.
Kaplan, J. O., Krumhardt, K.M. and Zimmermann, N. 2009. The prehistoric and preindustrial deforestation of Europe. Quaternary Science Reviews 28: 3016-3034.
King, F.H. 1911. Farmers of Forty Centuries, Or Permanent Agriculture in China, Korea and Japan. CreateSpace Independent Publishing Platform, Scots Valley, CA, USA.
McConnell D. 1935. The Chilean Nitrate Industry. Journal of Political Economy, 43(4): 506-529.
Nolan B.T., Ruddy B.C., Hitt K.J., and Helsel D.R. 1998. A National Look at Nitrate Contamination of Ground Water. Retrieved from http://water.usgs.gov/nawqa/nutrients/pubs/wcp_v39_no12/ on October 15, 2015.
Rockström J, Steffen W, Noone, K, Persson Å, Chapin S, Lambin E, Lenton TM, Scheffer , Folke C, Schellnhuber HJ, Nykvist B, de Wit CA, Hughes T, van der Leeuw S, Rodhe H, Sörlin S, Snyder PK, Costanza R, Svedin U, Falkenmark M, Karlberg L, Corell RW, Fabry VJ, Hansen J, Walker B, Liverman D, Richardson K, Crutzen P, and Foley J. 2009. Planetary Boundaries: Exploring the Safe Operating Space for Humanity. Ecology and Society 14(2): 32.
Seuss, Dr. 1971. The Lorax. Random House, New York, NY, USA.
Smil, V. 1999. Detonator of the population explosion. Nature 400: 415.
Smil, V. 1997. Global Population and the Nitrogen Cycle. Scientific American July: 76-81.
Speijers G.J.A. and Fawell J.K. 2011. Nitrate and nitrite in drinking-water. WHO Press, World Health Organization, Geneva, Switzerland. Retrieved from www.who.int/water_sanitation_health/dwq/chemicals/nitratenitrite2ndadd.pdf on October 15, 2015.
Steffen W., Richardson K., Rockström J., Cornel S.E., Fetzer I., Bennett E.M., Biggs R., Carpenter S.R., de Vries W., de Wit C.A., FolkeC., Gerten D., Heinke J., Mace G.M., Persson L.M., Ramanathan V., Reyers B., Sörlin S. 2015. Planetary boundaries: Guiding human development on a changing planet. Science 347: 736-346.
The Millennium Development Goals Report 2015. Retrieved from www.un.org/millenniumgoals/2015_MDG_Report/pdf/MDG%202015%20rev%20%28July%201%29.pdf on October 15, 2015.
USDA Economic Research Service: Food expenditures. Retrieved from www.ers.usda.gov/data-products/food-expenditures.aspx on November 5, 2015.
USEPA. 2015. Overview of Greenhouse Gasses. Retrieved from http://epa.gov/climatechange/ghgemissions/gases/n2o.html on October 15, 2015.
Vizcarra C. Guano, Credible Commitment, and Sovereign Debt Repayment in Nineteenth-Century Peru. The Journal of Economic History, 69(2): 358-387.
Notes
[1] In 1961, Sir William Crookes discovered thallium, a very toxic trace element. For a number of years I have researched how crop species can accumulate trace elements (including thallium) into their roots and shoots.
[2] Hager, p 4.
[3] Rockström, et al., 2009.
[4] Steffen, et al., 2014.
[5] Nitrogen compounds were known long before this; Rutherford isolated nitrogen gas from air, and soon after nitrogen was recognized as one of the elements. In the mid 1800’s, nitrogen was shown to be required by plants, and that its deficiency in soil limited plant growth.
[6]Photo retrieved from https://500m2.files.wordpress.com/2012/02/edamame-root-nodules.jpg on October 15, 2015.
[7] King, p. 50.
[8] Trager, p 53..
[9] Kaplan, et. al., 2009.
[10] Smil, 1997.
[11] Hager, p. 6.
[12] Hager, pp. 25-36.
[13] Vizcarra, 2009.
[14] Photos retrieved from www.southamericaliving.com/wp-content/uploads/ 2012/10/paracas_guano_making_550.jpg (left) and www.doomsteaddiner.net/forum/index.php?topic=1777.0 (right) on October 15, 2015.
[15] McConnell, 1935.
[16] BASF stands for Badische Anilin- und Soda-Fabrik, and is still in existence. It was founded in 1865 to make synthetic dyes. Aniline (which can be extracted from coal tar) is a precursor needed to synthesize dyes. German chemical companies helped to launch the modern chemical industry and developed a number of notable products, including dyes, ammonia, and the first sulfa-based antibiotics.
[17] Smil, 1999.
[18] Photos retrieved from www.sciencemuseum.org.uk/onlinestuff/People/Fritz%20Haber.aspx (left), and www.britannica.com/biography/Carl-Bosch (right) on October 15, 2015.
[19] Bown, p. 34.
[20] Bown, p. 34.
[21] In physics, chemistry, medicine, literature, and peace.
[22] Photo retrieved from http://peacelab.org/peace-pioneers/alfred-nobel/ on October 15, 2015.
[23] Photo retrieved from www.in-pharmatechnologist.com/Ingredients/Cure-not-kill-Dynamite-ingredient-can-improve-cancer-treatment-says-study on October 15, 2015.
[24] Brown, p. 81
[25] Smil, 1999.
[26] Smil, 1997.
[27] Retrieved from http://esa.un.org/unpd/wpp/ on October 15, 2015.
[28] Fowler et al. 2013.
[29] Fowler et al. 2013.
[30] Berry, p xiii.
[31] Galloway and Cowling, 2002.
[32] USEPA, 2015.
[33] Speijers and Fawell, 2011.
[34] Nolan et al., 1998.
[35] Steffen et al., 2015 (Supplemental material).
[36] Retrieved from http://www.wri.org/media/maps/eutrophication/fullscreen.html on October 15, 2015.
[37] Retrieved from www.google.ca/maps/@43.2785675,-79.8873368,4385m/data=!3m1!1e3 on October 15, 2015.
[38] Crouch, p 13.
[39] USDA-ERS: Food expenditures. Accessed from www.ers.usda.gov/data-products/food-expenditures.aspx on Nov 5th, 2015.
[40]The Millennium Development Goals Report 2015; p 4.
[41] Steffen et al, 2015.
[42] Holy Bible, NIV
[43] Dr. Seuss, the Lorax.
[44] Retrieved from www.uni-leipzig.de/ru/bilder/aufersth/b3-59.jpg on October 15, 2015. Note the tools of many gardeners – the straw hat, shovel, and pruning knife.